Lewis Dot Structure For Cl2
ClOtwo – (chlorite) has one chlorine atom and two oxygen atoms.
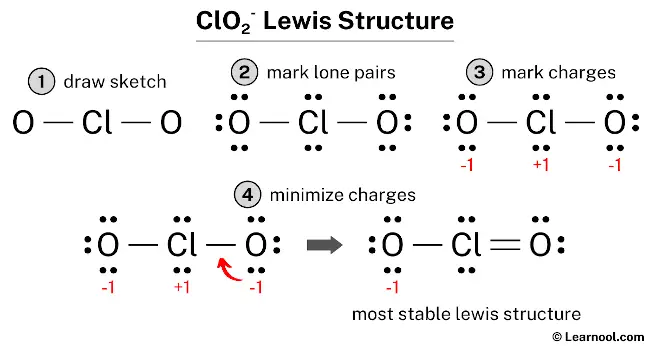
In the lewis structure of ClO2 –, there is one single bond and one double bond effectually the chlorine cantlet, with two oxygen atoms attached to it. The oxygen cantlet with a unmarried bail has three solitary pairs, and the oxygen cantlet with a double bond has two lone pairs.
Also, in that location is a negative (-i) charge on the oxygen atom with a single bail.
Steps
Here's how you can draw the ClO2 – lewis structure pace by stride.
Step #1: draw sketch
Step #2: mark lone pairs
Footstep #3: mark charges
Step #iv: minimize charges
Step #5: minimize charges again (if there are)
Allow's interruption down each footstep in detail.
#ane Describe Sketch
- Starting time, determine the full number of valence electrons
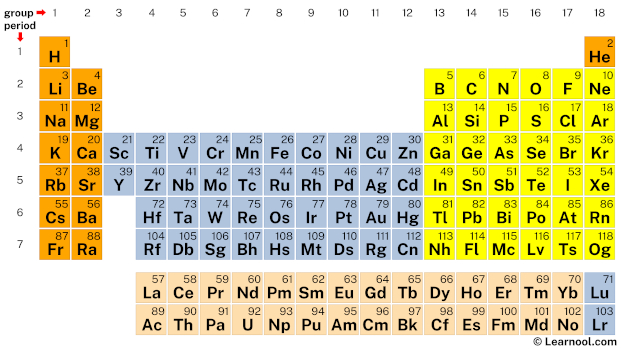
In the periodic table, chlorine lies in group 17, and oxygen lies in group 16.
Hence, chlorine has seven valence electrons and oxygen has six valence electrons.
Since ClOtwo – has one chlorine cantlet and ii oxygen atoms, so…
Valence electrons of one chlorine atom = vii × 1 = 7
Valence electrons of 2 oxygen atoms = six × 2 = 12
Now the ClO2 – has a negative (-1) charge, so we take to add together one more than electron.
So the total valence electrons = vii + 12 + i = xx
Learn how to discover: Chlorine Valence Electrons and Oxygen Valence Electrons
- Second, discover the total electron pairs
Nosotros take a total of 20 valence electrons. And when we carve up this value by two, we get the value of total electron pairs.
Total electron pairs = total valence electrons ÷ 2
So the total electron pairs = 20 ÷ ii = 10
- Third, determine the central atom
We have to place the to the lowest degree electronegative cantlet at the centre.
Since chlorine is less electronegative than oxygen, assume that the central cantlet is chlorine.
Therefore, place chlorine in the eye and oxygens on either side.
- And finally, describe the rough sketch
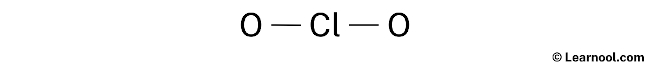
#2 Mark Alone Pairs
Hither, we accept a full of 10 electron pairs. And two Cl — O bonds are already marked. So nosotros have to only mark the remaining eight electron pairs as lone pairs on the sketch.
Also remember that chlorine is a menstruation 3 element, and so it can go along more than than eight electrons in its terminal shell. And oxygen is a period 2 chemical element, so it can not keep more than eight electrons in its last vanquish.
E'er start to marker the lonely pairs from outside atoms. Here, the outside atoms are oxygens.
So for each oxygen, there are 3 lone pairs, and for chlorine, there are two lonely pairs.
Marking the lone pairs on the sketch every bit follows:
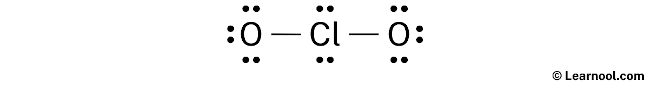
#iii Mark Charges
Use the post-obit formula to calculate the formal charges on atoms:
Formal charge = valence electrons – nonbonding electrons – ½ bonding electrons
For chlorine cantlet, formal accuse = seven – iv – ½ (4) = +1
For each oxygen atom, formal charge = vi – 6 – ½ (2) = -1
Here, both chlorine and oxygen atoms have charges, so mark them on the sketch as follows:
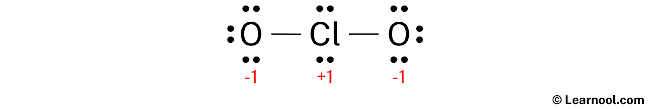
The to a higher place structure is not a stable lewis structure because both chlorine and oxygen atoms accept charges. Therefore, reduce the charges (as beneath) past converting lonely pairs to bonds.
#iv Minimize Charges
Convert a lonely pair of the oxygen atom to make a new Cl — O bond with the chlorine atom as follows:
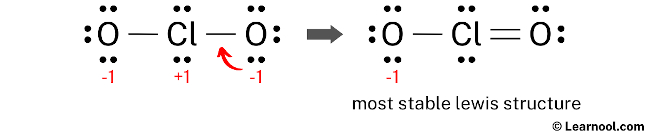
In the above structure, you can see that the primal atom (chlorine) forms an octet. And the outside atoms (oxygens) also form an octet. Hence, the octet rule is satisfied.
Now there is still a negative (-1) charge on the oxygen cantlet.
This is okay, considering the structure with a negative charge on the near electronegative atom is the all-time lewis structure. And in this case, the well-nigh electronegative element is oxygen.
Besides, the higher up structure is more stable than the previous structures. Therefore, this structure is the well-nigh stable lewis structure of ClOii –.
And since the ClOtwo – has a negative (-one) charge, mention that charge on the lewis structure by drawing brackets as follows:
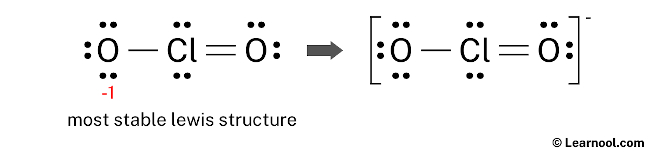
Next: ClO3 – Lewis Structure
.
.
.
External Links:
- https://techiescientist.com/clo2-lewis-construction/
- https://socratic.org/questions/how-tin can-i-depict-the-lewis-structure-for-clo2
- https://topblogtenz.com/chlorite-ion-clo2-lewis-construction-molecular-geometry-hybridization-polar-or-nonpolar/
- https://world wide web.thegeoexchange.org/chemistry/bonding/Lewis-Structures/ClO2-lewis-structure.html
- https://lambdageeks.com/clo2-lewis-structure/
Lewis Dot Structure For Cl2,
Source: https://learnool.com/clo2-lewis-structure/
Posted by: durstconage.blogspot.com


0 Response to "Lewis Dot Structure For Cl2"
Post a Comment